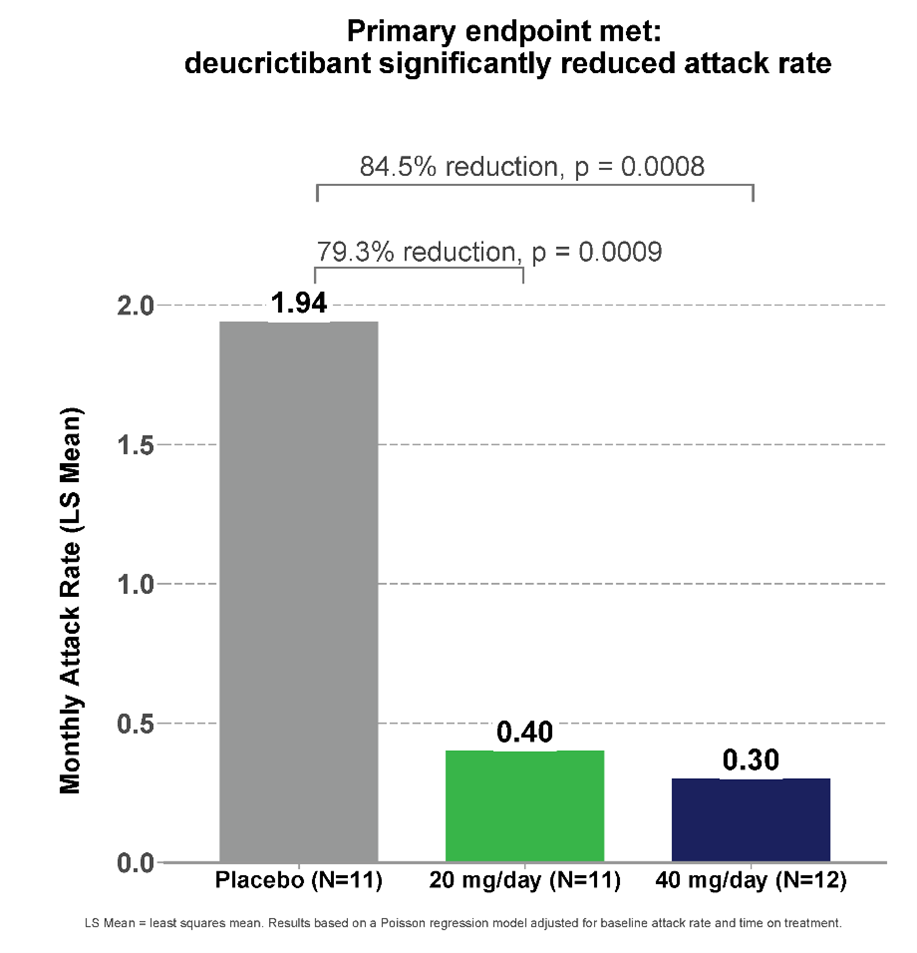
- The primary endpoint was met; Deucrictibant administered orally at 40 mg/day significantly reduced the average monthly attack rate by 84.5% (p=0.0008) compared to placebo.
- 92.3% reduction in moderate to severe attacks
- 92.6% fewer attacks by participants treated with on-demand medication
- Diuretics are well tolerated
- Pharvaris will host a conference call this morning at 8:00 AM EST
ZUG, Switzerland, Dec. 06, 2023 (GLOBE NEWSWIRE) — Pharvaris (Nasdaq: PHVS ), a clinical-stage company developing a novel, oral bradykinin B2 receptor antagonist for the treatment and prevention of hereditary angioedema, today announced positive attacks (HAE). . Line data from the CHAPTER-1 phase 2 clinical study met its primary endpoint, demonstrating statistically significant and clinically meaningful results of deucrictibant as oral preventive therapy for people living with HAE. Pharvaris plans to present data from the study at future medical meetings.
Chapter-1 Clinical Study Design and Results
CHAPTER-1 (NCT05047185) is a double-blind, placebo-controlled phase 2 study evaluating the efficacy, safety and tolerability of deucrictibant for long-term prophylaxis against angioedema attacks in HAE-1/2. In the study, 34 participants were enrolled globally and randomized to receive one of two doses of duractibant (20 mg/day or 40 mg/day) or placebo for 12 weeks of treatment. The Deucrictibant Immed-Release capsule (PHVS416) is dosed twice daily as a proof-of-concept for the formulation intended for the prophylactic treatment of HAE, the Daily Deucrictibant Extended-Release Tablet (PHVS719). The open-label portion of the CHAPTER-1 study was conducted at a dose of 40 mg/day.
The study’s primary endpoint measured the time-averaged number of investigator-confirmed HAE attacks during the treatment period. The monthly attack rate was reduced by 84.5% (p=0.0008) in participants who received deucrictibant 40 mg/day compared to placebo.
Marc A. Riedl, MD, MS, Professor of Medicine, Clinical Director of the American Hereditary Angioedema Association (HAEA) Angioedema Center at the University of California San Diego (UCSD), Chief of Clinical Services for Allergy/Immunology at UCSD, and Principal Investigator of the CHAPTER-1 study , commenting, the HAE community is searching for highly effective, well-tolerated, and low-burden therapies. The CHAPTER-1 data represent an important step forward in the evolution of HAE treatment. Given these encouraging results, deucrictibant has the potential to significantly improve clinical outcomes for people living with HAE.
Peng Lu, MD, Ph.D., Chief Medical Officer of Pharvaris, stated that Deucrictibant is the first HAE treatment capable of combining the efficacy and favorable safety profile of an injectable with the convenience of an oral regimen. The study shows for the first time that antagonism of the bradykinin B2 receptor can provide rapid and sustained protection from HAE attacks, including a significant reduction in moderate and severe attacks, including a clinically meaningful improvement in health-related quality of life. We hope to advance the development of deucrictibant to prevent HAE attacks.
Berndt Modig, CEO of Pharvaris added, “We sincerely thank the clinical trial participants and their guardians, the site investigators and staff, the HAE community, and the Pharvaris team for their contributions to the CHAPTER-1 study.” These study results, along with compelling data from our on-demand program, further strengthen our belief that deucrictibant may become the preferred option for both the treatment and prevention of HAE attacks.
In analysis of secondary endpoints, deucrictibant demonstrated a clinically meaningful improvement in attack severity and a reduction in the number of attacks treated with on-demand medication. Participants treated with ducritibant experienced a meaningful improvement in their quality of life. The table below lists additional study findings:
| Placebo N=11 |
20 mg/day N=11 |
40 mg/day N=12 |
||
| Monthly Attack Rate LS Mean (95% CI)* | ||||
| Moderate or severe attacks | 1.50 (0.91, 2.50) | 0.26 (0.08, 0.81) | 0.12 (0.02, 0.67) | |
| Attacks are treated with medication on demand | 1.41 (0.88, 2.24) | 0.35 (0.14, 0.85) | 0.10 (0.02, 0.57) | |
| Achieving a reduction in attack rate threshold at baseline** | ||||
| >=50% reduction | 2/11 (18%) | 9/11 (82%) | 9/10 (90%) | |
| >=70% reduction | 2/11 (18%) | 8/11 (73%) | 8/10 (80%) | |
| >=90% reduction | 0 | 6/11 (55%) | 6/10 (60%) | |
| Hit for free during treatment | 0 | 6/11(55%) | 4/10 (40%) | |
| LS = least squares; CI = confidence interval. *Results of monthly attack rates are based on Poisson regression models adjusted for baseline attack rate and time to treatment. Nominal p-value < 0.01 for all endpoints included in this section comparing deucrictibant with placebo. **Participants with 4 weeks of treatment (two participants at 40 mg/day) achieving a threshold reduction in attack rate from baseline were not included in the summaries of proportions. All endpoints included in this section had a nominal p-value <0.05 compared with placebo. |
||||
Throughout the 12-week treatment period in phase 1, both doses of the diuretic were well tolerated. Serious adverse events, severe treatment-emergent adverse events, and no adverse events leading to treatment discontinuation.
In August 2022, the US Food and Drug Administration (FDA) suspended clinical studies of deucrictibant, including CHAPTER-1. Favaris notified specific regulatory authorities in Canada, Europe, Israel, and the United Kingdom regarding its clinical hold in the United States, and the regulatory status of Deucrictibant outside the United States has not been affected. In June 2023, Favaris announced that it would lift the clinical hold for the on-demand treatment of HAE in the U.S. Favaris has completed the FDA-required 26-week rodent toxicology study, which we believe was accomplished. let’s Pharvaris plans to submit study results to the FDA by the end of the year. However, the nature or timing of the response from the FDA is not certain.
Conference call
Pharvaris will host a live conference call and webcast to discuss the high-line data from the CHAPTER-1 study in more detail via a live webcast this morning at 8:00 EST. The presentation slides can be accessed on the Events and Presentations page of the Pharvaris Investor Relations website. Participants interested in asking a verbal question during the Q&A may do so during the live conference call. An archived replay is also available on the website for 90 days after the event.
About Deucrictibant
Deucrictibant is a potent, selective and orally available antagonist of the bradykinin B2 receptor. By inhibiting bradykinin signaling through the bradykinin B2 receptor, durectibant has the potential to treat the clinical signs of an HAE attack and prevent attacks from occurring. Based on its chemical properties, Pharvaris is developing two deucrictibant compounds for oral administration; A capsule for rapid onset of action in acute treatment and an extended-release tablet to enable sustained absorption and efficacy in prophylactic treatment.
About Favaris
Building on its deep-rooted roots in HAE, Pharvaris is a clinical-stage company developing a novel, oral bradykinin B2 receptor antagonist for the treatment and prevention of HAE attacks. By directly pursuing this clinically proven therapeutic target with new small molecules, the Pharvaris team hopes to develop an efficient, safe, and easily administered treatment for attacks, both on-demand and prophylactically, for people with all subtypes of HAE. To provide options. The company brings together the best talent in the industry with deep expertise in rare diseases and HAE. For more information, visit https://pharvaris.com/.
Forward-looking statements
This press release contains certain forward-looking statements that involve significant risks and uncertainties. All statements that do not relate to historical facts contained in this press release should be considered forward-looking statements, including, without limitation, statements relating to our future plans, studies and experiments and any statements containing the words believe, expect, Hopes, estimates, may, could, should, would, will, intend and similar expressions. These forward-looking statements are based on management’s current expectations, are not promises or guarantees, and involve known and unknown risks, uncertainties and other important factors that could cause Pharvaris’ actual results, performance or achievements to be materially different from its stated or implied expectations. May cause change. By way of forward-looking statements. Such risks include, but are not limited to, the following: the uncertainty of the outcome of our interactions with regulatory authorities, including the FDA, regarding the clinical retention of the prophylactic deucractivant in the United States; the expected timing, progress or success of our clinical development programs, in particular PHVS416 (immediate-release dehydrating capsules) and PHVS719 (extended-release dehydrating tablets), which are currently in mid-stage global clinical trials; risks arising from pandemics, such as the COVID-19 pandemic, which could adversely affect our business, non-clinical studies and clinical trials; the expected timing and results of rodent toxicology studies and our ability to resolve any issues in a timely manner to the satisfaction of the FDA or any regulatory agency; timing of regulatory approvals; the value of our common stock; the time, cost and other constraints involved in obtaining regulatory approval for our product candidates PHVS416 and PHVS719, or other product candidates we may develop in the future; the ability to establish commercial capabilities or enter into agreements with third parties to market, sell and distribute our product candidates; our ability to compete in the pharmaceutical industry, including existing therapies, emerging competitive therapies and competing generic products; the ability to achieve marketing, commercialization and market acceptance for our product candidates; our ability to raise capital when needed and on acceptable terms; regulatory developments in the United States, the European Union and other jurisdictions; the ability to protect our intellectual property and to conduct our business without infringing on the intellectual property rights or regulatory exclusivity of others; our ability to manage the negative consequences of changes in applicable laws and regulations, including tax laws, our ability to successfully remediate material weaknesses in our internal control over financial reporting and our ability to maintain an effective system of internal control over financial reporting; changes and uncertainty in general market, political and economic conditions, including as a result of inflation and the current conflict between Russia and Ukraine and the Hamas attack on Israel and subsequent war; and other factors described under the headings Cautionary Statement Regarding Forward-Looking Statements and Items 3. Key InformationD. Form 20-F and the U.S. Risk factors in our annual report on periodic filings with the Securities and Exchange Commission. These and other important factors could cause actual results to differ materially from those indicated by the forward-looking statements presented in this press release. Any such forward-looking statements represent management’s estimates as of the date of this press release. New risks and uncertainties may emerge from time to time, and not all risks and uncertainties can be predicted. Although Pharvaris may choose to update such forward-looking statements at some point in the future, even if subsequent events cause it to change its views, Pharvaris disclaims any obligation to do so. These forward-looking statements should not be relied upon as representations made by Pharvaris at any time after the date of this press release.

#Pharvaris #Announces #Positive #Topline #Phase #Data #Chapter #Study #Deucrictibant #Prophylactic #Treatment #HAE #Attacks
Image Source : www.globenewswire.com